Comet assay in 3D human skin models
- Details
- Created: Wednesday, 20 August 2014 14:21
Researchers from across Europe and the USA have been working in collaboration to investigate inter- and intra-laboratory variation when performing the comet assay on reconstructed 3D human epidermal skin models.
The skin is the first site of contact for many compounds, including ingredients of beauty and household care products, agrochemicals, dermal pharmaceuticals and industrial chemicals.
Currently, rather than methods based on cells cultured in 2D, there is a trend towards the development of methods that use human 3D tissue equivalents and a representative route of exposure in toxicology studies. Reconstructed 3D human epidermal skin models are currently considered more relevant than cell lines because of their morphological resemblance to human skin.
These reconstructed 3D human epidermal skin models are being used increasingly for safety testing of chemicals and an assay was developed in which the tissues were topically exposed to test chemicals for three hours. Then the cells are isolated and DNA damage is assessed using the comet assay.
This study was split into two phases:
Phase 1
The inter-laboratory reproducibility of the 3D skin comet assay was initially demonstrated using two model genotoxic carcinogens (methyl methane sulfonate (MMS) and 4-nitroquinoline-n-oxide). The results showed good concordance among three different laboratories.
Phase 2
Intra- and inter-laboratory reproducibility was investigated by supplying each laboratory with five coded compounds, each with different genotoxicity.
For the genotoxic carcinogens (three compounds), all laboratories reported a dose-related and statistically significant increase in DNA damage in every experiment.
The non-genotoxic and non-carcinogenic compound showed an increase in DNA damage when compared to the solvent control in only one laboratory. However, the response was not dose related and the compound was judged negative overall.
Finally, a non-carcinogenic, genotoxic in vitro but not in vivo compound was the only compound showing clear cytotoxic effects. Here, significant DNA damage typically occurred only at doses that were substantially cytotoxic (>30% cell loss), and the overall response was comparable in all laboratories.
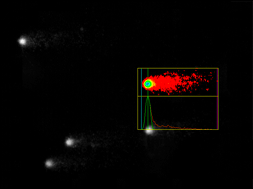 A traditional comet assay method was followed and the slides were coded to prevent operator bias during analysis of the slides. The slides were stained with SYBR gold and at least two slides per skin model were analysed. Fifty nuclei per slide were randomly measured with Comet Assay IV software (Perceptive Instruments, Suffolk, UK). Three to four regions of the gel were randomly selected to represent the whole slide and overlapping cells, cells close to the border of the slide and ghost cells were excluded from analysis.
A traditional comet assay method was followed and the slides were coded to prevent operator bias during analysis of the slides. The slides were stained with SYBR gold and at least two slides per skin model were analysed. Fifty nuclei per slide were randomly measured with Comet Assay IV software (Perceptive Instruments, Suffolk, UK). Three to four regions of the gel were randomly selected to represent the whole slide and overlapping cells, cells close to the border of the slide and ghost cells were excluded from analysis.
The researchers concluded that the results of the collaborative study for the coded compounds were generally reproducible among the laboratories involved and intra-laboratory reproducibility was also good. The scientists involved with this study concluded that these data indicate that the comet assay in skin models is a promising model for the safety assessment of compounds with a dermal route of exposure.
Case study based on:
Comet assay in reconstructed 3D human epidermal skin models--investigation of intra- and inter-laboratory reproducibility with coded chemicals.
Reus AA1, Reisinger K, Downs TR, Carr GJ, Zeller A, Corvi R, Krul CA, Pfuhler S. Mutagenesis. 2013 Nov;28(6):709-20.
